
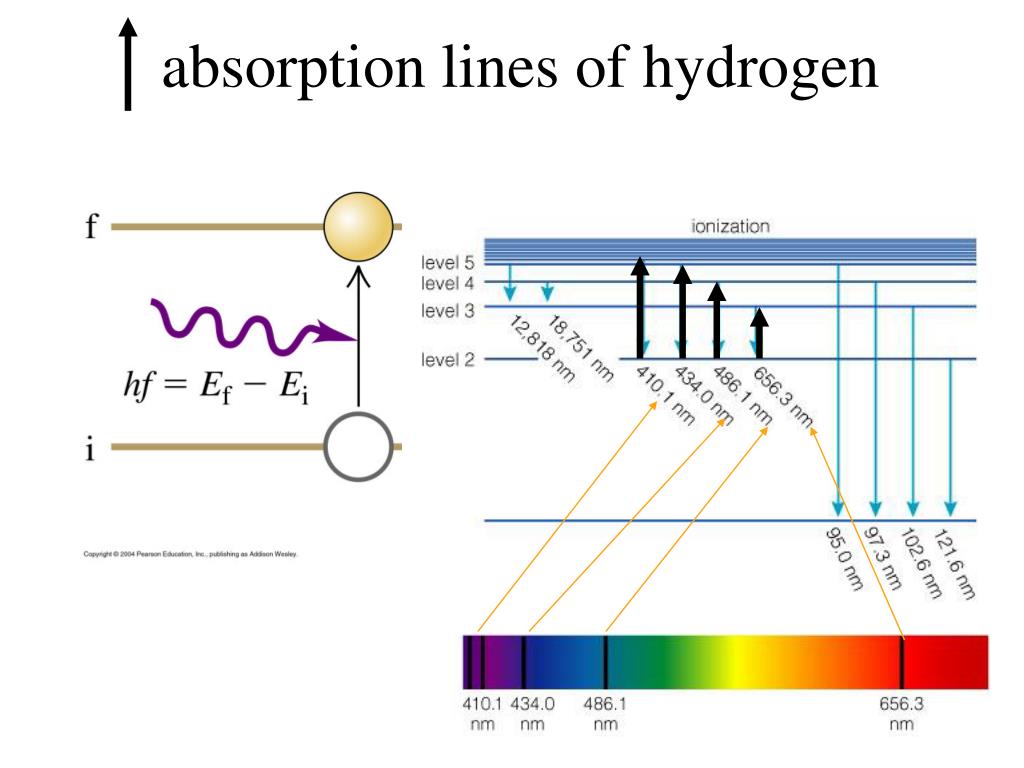
However, it is a major systematic uncertainty for the calibration of an IR absorption based concentration-measurement and requires a separate treatment. For this work, the precise ortho/para ratio is not needed for the discussion. 25 This ratio changes by natural equilibration, which can be neglected due to its long conversion times (>days 26,27). The J 1 to J 0 ratio is 3:1 for H 2 (6:3 for D 2) directly after the liquefaction. Therefore, the first excited rotational state J = 1 is occupied in the case of a sufficiently fast cool down. Due to the forbidden ortho ↔ para transition of the homonuclear isotopologues, the sum of the odd J states and the sum of even J states are almost constant in time. The set of descriptors found within this work can be used to identify and predict all lines in this range for liquid H 2–D 2mixtures.Īt low temperatures of about 20 K, almost only the ground state of the molecules is expected to be occupied. We show a detailed analysis of these three spectra in the first and second vibrational branch in the range from 2000 cm −1 to 9000 cm −1. To develop and test the set of descriptors, we make use of three spectra: a pure H 2 sample, a pure D 2 sample, and a mixed H 2–D 2 sample. In particular, molecular dimers contribute to the absolute number of absorption lines in the spectra of mixed isotopologues. For this, we grouped the absorption lines into three groups: absorption on monomers, phonons, and molecular dimers. As we aim for a system independent calibration of IR absorption spectroscopy against all six isotopologues and three ortho–para ratios, we need a minimal and complete set of descriptors to predict the spectra and to decrease the needed calibration effort. Therefore, the complexity tremendously increases with the number of different isotopologues in the sample. It is the basic unit of all light carrying the energy E= hf.Ītomic emission spectrum: is the pattern of lines corresponds to a different electron transition from a higher energy state to a lower energy state.The IR spectra of liquid hydrogen isotopologues (Q 2 = H 2, D 2, T 2, HD, HT, DT) are dominated by the interaction induced absorption. Photon: the smallest discrete amount of electromagnetic radiation. Every element has a unique atomic absorption and emission line spectrum.Įxcited-state: of an atom is a state where its potential energy is higher than the ground state.The atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains.In the second example below, where an element has 3 excited states, it could emit photons at 6 specific wavelengths/frequencies (ΔE= hf).

Every element has a unique atomic absorption and emission line spectrum.

An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains.Įach of these spectral lines corresponds to a different electron transition from a higher energy state to a lower energy state. When a narrow beam of this light was viewed through a prism, the light was separated into four lines of particular wavelengths. Scientists studied the distinctive pink color of the gas discharge created by hydrogen gas. Signs of other colors contain different gases or mixtures of gases. However, only signs that glow with the red-orange color seen in the figure are filled with neon. “Neon” signs are familiar examples of gas discharge tubes. Electrons in the gaseous atoms first become excited, and then fall back to lower energy levels, emitting light of a distinctive color in the process.
These gas discharge tubes are enclosed glass tubes filled with a gas at low pressure through which an electric current is passed.
Since the electron energy levels are unique for each element, every gas discharge tube will glow with a distinctive color depending on the identity of the gas. One way for atoms to gain energy is to pass an electric current through an enclosed sample of a gas at low pressure called a gas discharge tube.


 0 kommentar(er)
0 kommentar(er)
